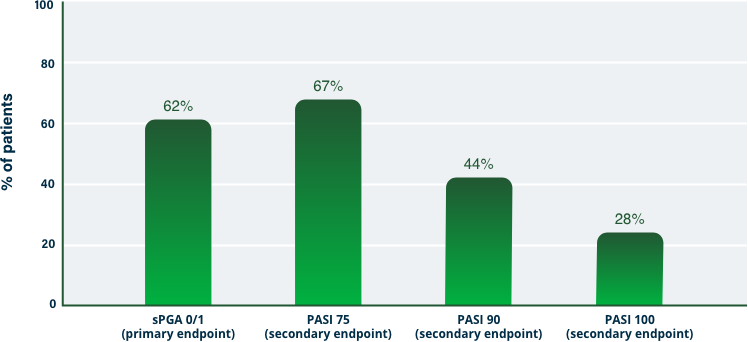
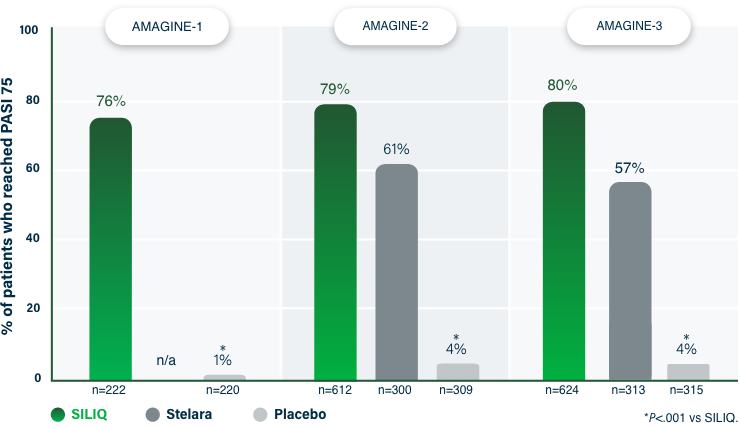
75% Clearance at Week 121-3
PASI 75 | Key endpoint | NRI analysis
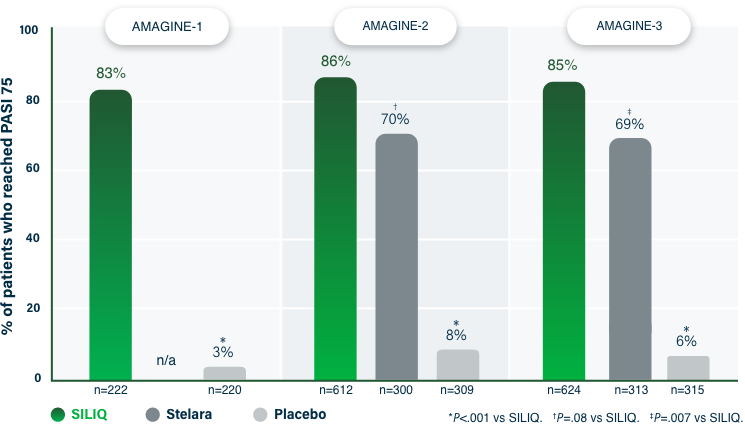
NRI, nonresponder imputation; PASI, Psoriasis Area and Severity Index.
sPGA (0/1) Results at Week 121-3
Key endpoint | NRI analysis
NRI, nonresponder imputation; sPGA, Static Physician’s Global Assessment
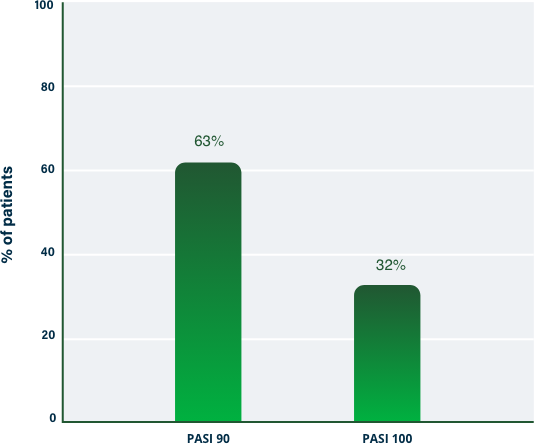
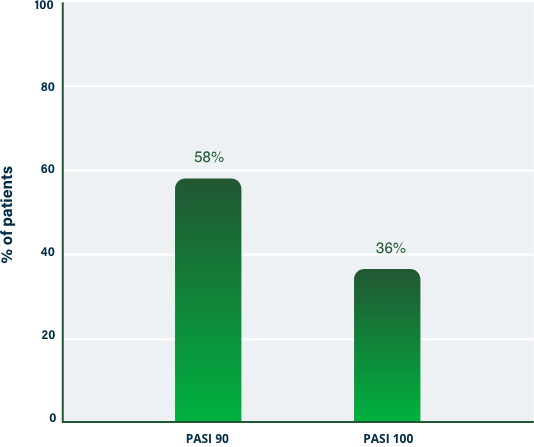
100% Clearance at Week 121-3
PASI 100 | Key endpoint | NRI analysis
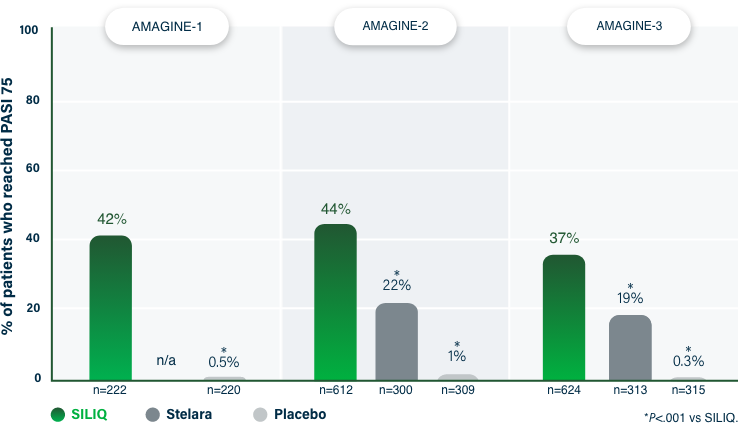
NRI, nonresponder imputation; PASI, Psoriasis Area and Severity Index.